Abstract
Background: Antimicrobial resistance (AMR) is a growing global threat, exacerbated by increasing antibiotic prescribing in secondary care. In haemato-oncology, 60% of deaths are infection-related, with multi-drug resistant bacteria associated with inferior overall survival. There is an urgent need to implement antimicrobial stewardship (AMS) interventions, balanced against infection-related morbidity and mortality.
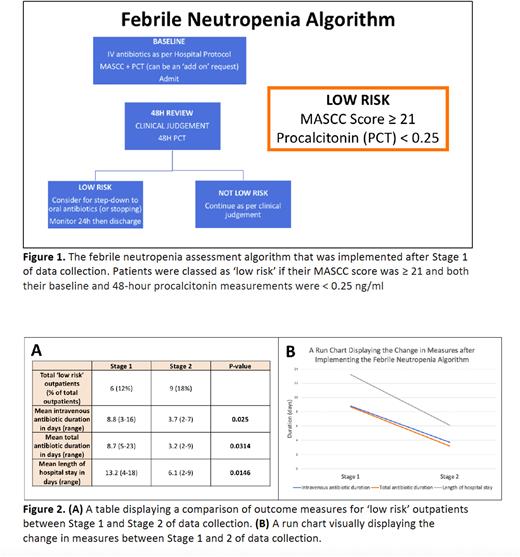
AMS tools include the Multinational Association for Supportive Care in Cancer (MASCC) score and the hormokine procalcitonin (PCT). To our knowledge, this is the first study to combine these tools to create a risk stratification model which identifies 'low risk’ patients with febrile neutropenia (Figure 1). We hypothesised that our treatment algorithm would allow robust risk stratification, reduce antibiotic duration and shorten length of hospital stay.
Objectives:
To assess whether a novel febrile neutropenia algorithm combining MASCC score and PCT allows robust risk stratification
To assess whether our febrile neutropenia algorithm reduces length of antibiotic treatment and hospital stay
Methods: This study consisted of two stages of data collection and took place in a single tertiary haemato-oncology centre in the UK between 2020-2022. The initial stage (n=49) was an observational study of patients with a new presentation of febrile neutropenia (neutrophils <1.0 x 109/L and temperature ≥38oC). MASCC score was calculated at baseline and PCT at baseline and 48 hours to classify each patient as either 'low risk’ or 'not low risk’ (Figure 1). Treating clinicians were not aware of these results. Following initial analysis, we introduced a flowchart for the investigation and management of febrile neutropenia (Figure 1). We completed stage two of data collection following this intervention (n=50).
For objective one, we combined all data (n=99) to appraise the use of our algorithm to successfully identify 'low risk’ patients. We calculated sensitivities and negative predictive values (NPV) in predicting blood stream infections (BSI), defined as an organism identified on blood cultures. We used Fishers exact test (2 tail) to investigate statistical significance.
For objective two, our primary endpoints were duration of antibiotics and hospital length of stay. We compared these endpoints between the two stages of data collection. The two-sample T-test assessed statistical significance between each stage.
Results: 25 out of 99 febrile neutropenia patients (25.3%) had a BSI. Our risk stratification model had a sensitivity of 92.0% and NPV of 92.6% in detecting BSI (p=0.0103). 2 out of 27 (7.4%) patients defined as 'low risk’ had a BSI. Both patients were inpatients, remained clinically stable, and grew staphylococcus haemolyticus and streptococcus agalactiae respectively.
More outpatients were classified as 'low risk’ compared to inpatients (15/50 (30%) versus 9/49 (18%) respectively). The majority of low-risk outpatients had lymphoma (8/15 (53.3%)). BSI were more commonly seen in inpatients compared to outpatients (21/49 (42.9%) versus 4/50 (8%) respectively), highlighting differing disease characteristics. 9 out of 50 (18%) outpatients with febrile neutropenia were identified as 'low-risk'. None of these patients had a BSI.
For objective two, we exclusively focussed risk stratification on outpatients with febrile neutropenia. Following implementation of our algorithm, there was a statistically significant reduction in duration of intravenous antibiotics (8.7 to 3.2 days, p=0.0314), total duration of antibiotics (13.2 to 6.1 days, p=0.0146) and length of hospital stay (8.8 to 3.7 days, p=0.0250) in 'low-risk’ outpatients (Figure 2).
Conclusion: Combining MASCC score and PCT allows robust risk stratification in outpatients presenting with febrile neutropenia and significantly reduces antibiotic duration and length of hospital stay. The majority of these patients had lymphoma. In addition to promoting AMS, our tool provides economic benefits and could improve patient quality of life.
We plan to rationalise antibiotic use further in low-risk outpatients by prescribing oral antibiotics from the outset with a period of hospital admission to ensure safety. Morbidity outcomes including re-admission rates will be measured. We hope this will drive down antibiotic use and length of stay further without compromising patient safety.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal